Smart-FDA Webinar: how Generative AI helps the pharmaceutical and life sciences industry stay ahead of regulatory requirements
On Tuesday, Jan 26, we hosted our first webinar on Smart-FDA, where we covered what takes place behind the scenes and how Generative AI helps the pharmaceutical and life sciences industry stay ahead of regulatory requirements.
We walked through some of the key features & benefits:
✅ Proactive Compliance: Facilitates compliance awareness and encourages a preventative approach, helping companies avoid costly penalties and reduce compliance risk.
✅ Extensive Database: Includes all FDA 483 inspection reports published by the FDA over the past 15 years, enabling comprehensive historical insights.
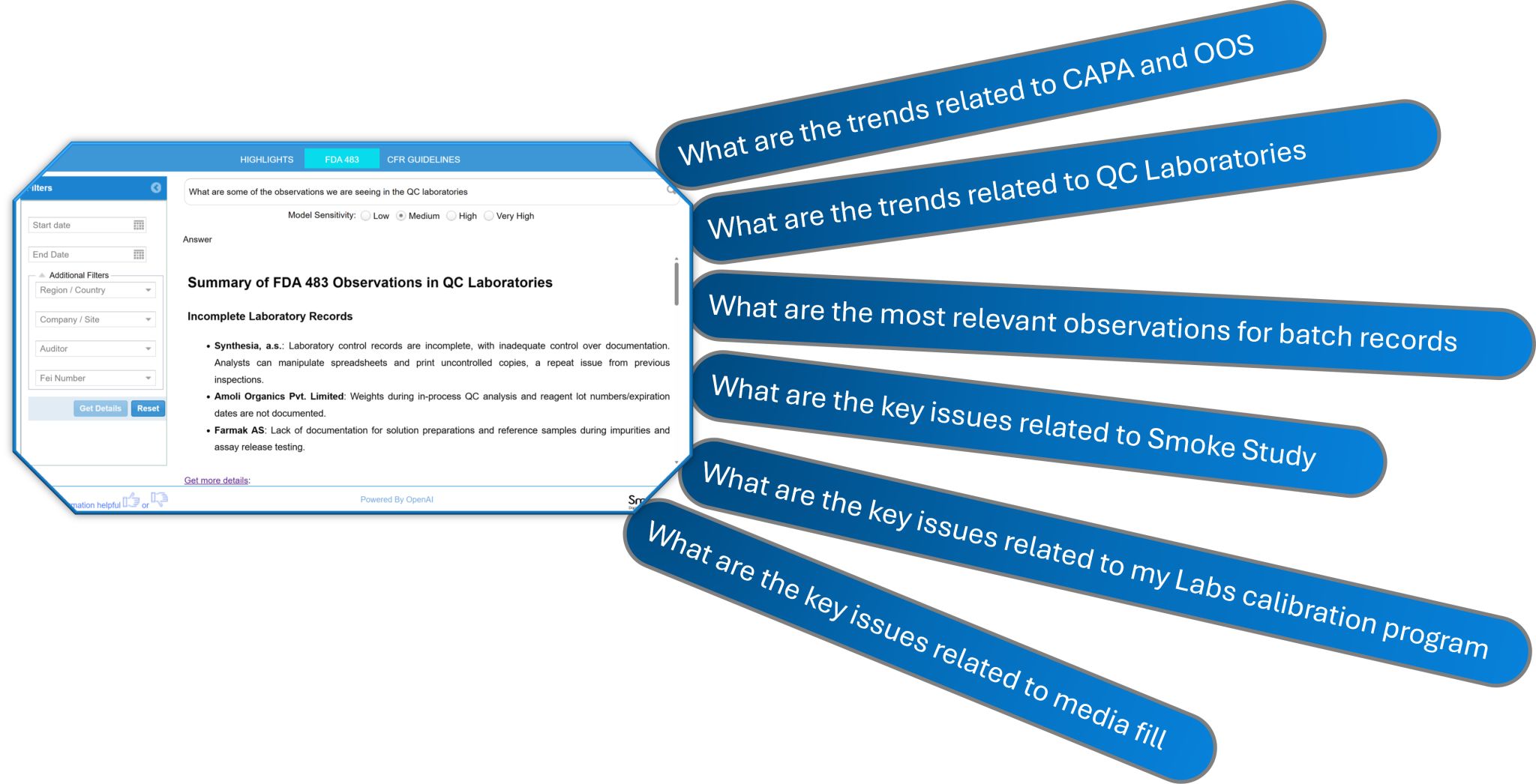
✅ Conversational Search: Users can query the database naturally by asking questions and receiving precise, context-aware answers.
✅ Integrated Modules: Bundled with Smart-QC & Smart-QA, providing a complete ecosystem for quality and compliance management.
We also discussed how customized prompts for each industry role allow users to leverage Smart-FDA to enhance knowledge specific to their area of responsibility. One of the most exciting parts of Smart-FDA is the Natural Language Search & Intelligent Discovery, which allows users to interact with the system using natural language; similar to ChatGPT, but powered by our extensive, vectorized FDA 483 database.
Lastly, we emphasized that by harnessing advanced AI capabilities, organizations can significantly improve how they identify and manage compliance risks.
Smart-FDA integrates multiple information sources with AI-driven analytics to:
✅ Equip companies with insights needed to manage risk proactively
✅ Prepare for inspections by highlighting areas historically identified by the FDA as critical
✅ Help compliance teams focus on high-priority issues, maximizing impact
When resources, time, and budgets are limited, leveraging technology, advanced algorithms, and analytics become essential to minimize risk and ensure regulatory alignment. Let us know what you think; we’d be delighted to schedule a demo with you and your team.
