Stability Center of Excellence: YES, or NO?
Pharmaceutical companies often face a key decision:
- Should stability sample testing be conducted at the manufacturing site, or 2. Should a dedicated Stability Center of Excellence be established?
This question parallels the ongoing debate about whether stability testing should be handled separately from release testing. However, the discussion goes beyond efficiency—it can significantly impact compliance.
Let’s explore both perspectives:
✅ Why Keep Stability Testing at the Manufacturing Site?
- Efficiency in Campaigning: Testing stability samples alongside release samples offers more efficient campaigning, as we often align stability tests with release testing cycles.
- Larger Team Flexibility: A bigger team at the manufacturing site provides flexibility in resource management. If handled well, it can shift resources without jeopardizing service level and compliance
- Lower Costs: It eliminates the need for a dedicated lab, management team, and supervisors. The result? A more cost-effective solution.
- Better Equipment Utilization: The same equipment used for release can be maximized for stability testing.
- Reduced Logistics Costs: Shipping samples to a remote center of excellence would incur additional costs.
✅ Key Arguments for a Stability Center of Excellence
- Compliance: A primary driver for this option is the concern over compliance. In some cases, business pressures lead to prioritizing release testing over stability testing, causing missed testing windows.
- Predictability: A dedicated center of excellence offers a more focused environment, where stability sample testing is scheduled and executed with predictability.
- Centralized Resource Allocation: If multiple manufacturing sites send samples to a central facility, there may be limitations in campaign efficiency, but the benefit of ensuring compliance and a dedicated focus may outweigh the challenges.
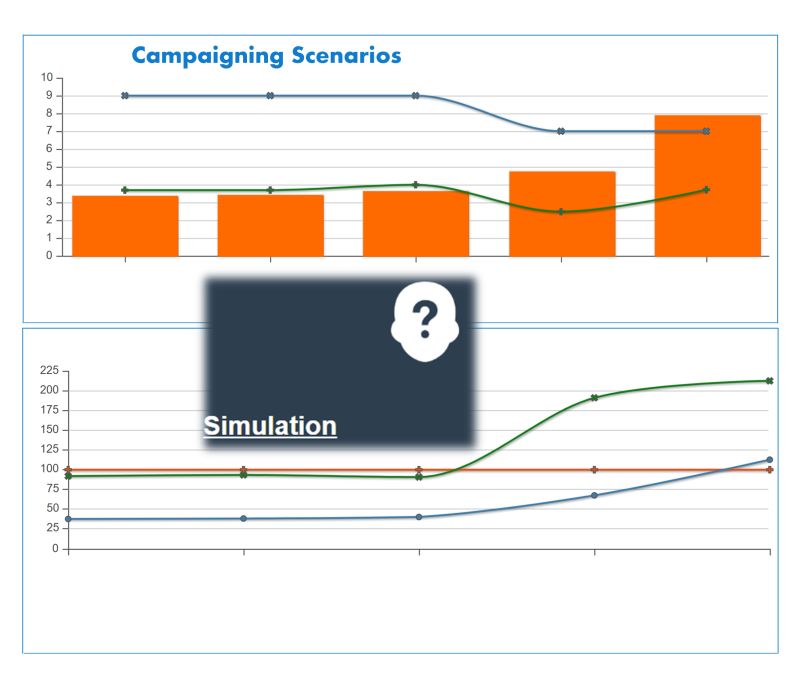
Mitigating Efficiency Loss in a Centralized Model
✅ Smart-QC Simulation – Quantifies the additional hours required due to reduced campaigning.
✅ Smart-QC Scheduling & Expediting – Optimizes testing windows and minimizes delays to maintain efficiency.
In Summary
✅ If a manufacturing site has not faced significant compliance issues with stability testing, the need for separation may be minimal. However, if compliance concerns persist, establishing a Stability Center of Excellence could be a strategic solution.
✅ The decision ultimately comes down to balancing compliance needs with operational efficiency.
💬 What’s your take? Is Stability Center of Excellence the way forward? Let’s discuss!
